Оглавление
- Введение в подготовку образцов для рентгеновского анализа
- Размер частиц: Основа успешной подготовки гранул
- Загрязнение: Тихий саботажник в рентгенофлуоресцентном анализе
- Выбор связующего: ключ к однородным гранулам
- Толщина гранул: Обеспечение достаточной глубины отбора проб
- Применение давления: Баланс между сжатием и однородностью
- Коэффициент разбавления: Тонкая настройка для получения точных результатов
- Перекрестное загрязнение пробы от пробы к пробе: Предотвращение интерференции
- Лучшие практики по снижению ошибок при подготовке гранул для рентгеновского анализа
- Заключение: Достижение точности в рентгенофлуоресцентном анализе
Введение в подготовку проб для рентгенофлуоресцентного анализа
В сфере рентгенофлуоресцентного анализа (РФА) точность подготовки проб имеет первостепенное значение для получения точных и надежных результатов. Прессование гранул играет решающую роль в этом процессе, обеспечивая гомогенизацию образцов и их готовность к анализу. Однако такие распространенные проблемы, как неправильный размер частиц, загрязнение, неправильный выбор связующего и недостаточное давление, могут привести к значительным ошибкам. В этой статье рассматриваются наиболее часто встречающиеся "подводные камни" при подготовке образцов на PELLET PRESS XRF и предлагаются практические решения для их преодоления, что в конечном итоге поможет вам достичь наивысшего уровня точности при проведении XRF-анализа.
Размер частиц: Основа успешной подготовки гранул
Размер частиц является критическим фактором при подготовке прессованных гранул, существенно влияющим на точность и надежность результатов анализа. Достижение идеального размера частиц, обычно менее 50 мкм, важно по нескольким причинам. Во-первых, частицы меньшего размера обеспечивают лучшую однородность образца. Если частицы слишком велики или отличаются по размеру, это может привести к несоответствию в распределении элементов в грануле, что может привести к искажению аналитических данных.
Важность равномерного размера частиц
Равномерный размер частиц имеет первостепенное значение по нескольким причинам. Более мелкие частицы обеспечивают более эффективное уплотнение и связывание при прессовании гранул. Такая однородность обеспечивает равномерное сжатие образца, что приводит к получению более стабильных и надежных результатов анализа. В отличие от этого, более крупные частицы или частицы разного размера могут создавать неоднородность в грануле, когда разные участки гранулы могут содержать разные концентрации элементов. Такая неоднородность может привести к ошибочным показаниям, поскольку аналитическое оборудование может неравномерно пробовать всю гранулу.
Достижение идеального размера частиц
Чтобы добиться идеального размера частиц менее 50 мкм, можно использовать различные лабораторные методы. Обычные методы включают использование дробилок, измельчителей и мельниц для уменьшения размера частиц образца перед прессованием. Обычно считается приемлемым диаметр частиц 40 мкм или ниже. Эти методы обеспечивают тонкое измельчение образца, что позволяет добиться лучшей однородности и консистенции конечного гранулята.
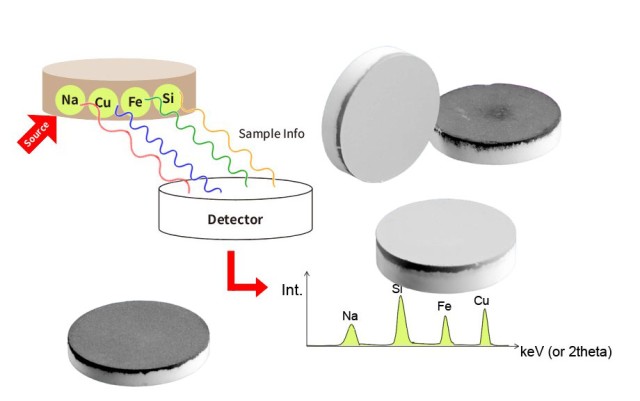
Влияние размера частиц на результаты анализов
Влияние размера частиц на результаты анализа трудно переоценить. Меньший размер частиц имеет решающее значение для получения гранул, обеспечивающих наилучшие аналитические результаты. Например, в рентгенофлуоресцентном анализе (XRF) глубина отбора проб или критическая глубина выхода элементов из образца зависит от энергии. Элементы с большей длиной волны, такие как натрий (Na), имеют меньшую глубину выхода, чем элементы с меньшей длиной волны, такие как железо (Fe). Это означает, что при анализе Na берется проба только первых 10 мкм образца. Поэтому любые неоднородности на этой небольшой глубине могут существенно повлиять на точность анализа.
Напротив, более крупные или переменные размеры частиц могут привести к неоднородности образца. Эти неоднородности могут привести к непостоянному распределению элементов в грануле, что приведет к недостоверным аналитическим данным. Например, если гранулы содержат области с различными размерами частиц, аналитическое оборудование может неравномерно отбирать пробы из этих областей, что приведет к расхождениям в измеренных концентрациях элементов.
Лучшие практики контроля размера частиц
Для обеспечения наилучших результатов анализа необходимо следовать передовым методам контроля размера частиц. Это включает в себя использование соответствующих методов измельчения и размола для достижения однородного размера частиц менее 50 мкм. Внимание к деталям и последовательность в процессе подготовки также имеют решающее значение. Обеспечивая измельчение образца до идеального размера частиц, аналитики могут свести к минимуму риск возникновения неоднородностей и получить более точные и надежные результаты анализа.
В целом, размер частиц является основой успешной подготовки гранул. Достижение и поддержание равномерного размера частиц менее 50 мкм имеет решающее значение для обеспечения однородности образца, что, в свою очередь, приводит к получению более точных и надежных результатов анализа. Следуя передовому опыту и уделяя пристальное внимание процессу подготовки, аналитики могут значительно улучшить качество аналитических данных.
Загрязнение: Тихий саботажник в рентгенофлуоресцентном анализе
Загрязнение - одна из самых серьезных проблем в рентгенофлуоресцентном анализе (РФА), особенно в процессе пробоподготовки. Стадия измельчения - критическая точка, где легко может произойти загрязнение, приводящее к неточным и вводящим в заблуждение результатам. Понимание источников загрязнения, внедрение эффективных методов предотвращения и осознание последствий загрязнения очень важны для сохранения целостности рентгенофлуоресцентного анализа.
Источники загрязнения
Загрязнения в рентгенофлуоресцентном анализе могут возникать из различных источников, но особенно уязвим процесс измельчения. Во время шлифования в образец могут случайно попасть внешние компоненты из инструмента для подготовки пробы. Это может произойти, если шлифовальные инструменты или контейнеры не были должным образом очищены между пробами, что приводит к перекрестному загрязнению. Например, остатки предыдущих проб могут остаться на шлифовальных инструментах и попасть в новую пробу, изменив ее элементный состав.
Другим распространенным источником загрязнения является использование неподходящих материалов для подготовки проб. Например, если шлифовальные инструменты изготовлены из материалов, которые могут просыпать частицы, или если контейнеры не являются инертными, эти материалы могут привнести нежелательные элементы в образец. Кроме того, загрязнению могут способствовать такие факторы окружающей среды, как пыль, влажность и пары химических веществ в лаборатории.
Методы предотвращения загрязнения
Предотвращение загрязнения при проведении рентгенофлуоресцентного анализа требует тщательного внимания к деталям и соблюдения строгих протоколов. Один из наиболее эффективных методов - обеспечить тщательную очистку и стерилизацию всех шлифовальных инструментов и контейнеров перед каждым использованием. Для этого можно использовать растворители или специализированные чистящие средства, совместимые с анализируемыми материалами.
Другая стратегия заключается в использовании инертных материалов для шлифовальных инструментов и контейнеров. Такие материалы, как боросиликатное стекло или высокочистый глинозем, менее склонны к попаданию загрязняющих веществ в образец. Кроме того, важно работать в чистой среде, предпочтительно в специальной зоне подготовки проб, свободной от пыли и других потенциальных загрязнителей.
Для порошковых образцов очень важно тщательно подготовить кюветы и пленки. Существует множество типов пленок для XRF, и выбор наиболее подходящего типа для конкретной задачи и устройства очень важен. Консультация с поставщиком поможет убедиться, что выбранная пленка подходит для образца и не будет содержать загрязнений.

Последствия загрязнения для результатов рентгенофлуоресцентного анализа
Загрязнение может иметь серьезные последствия для результатов рентгенофлуоресцентного анализа, приводя к неточным и ненадежным данным. Если проба загрязнена в процессе измельчения, элементный состав, измеренный прибором XRF, будет неверным. Это может привести к ложным положительным или отрицательным результатам, неправильной интерпретации данных и, в конечном счете, к неверным выводам.
Например, если образец, предназначенный для анализа на содержание свинца, загрязнен кальцием из предыдущего образца, результаты рентгенофлуоресцентного анализа могут показать искусственно высокую концентрацию свинца. Это может привести к неправильной диагностике загрязнения свинцом, что может иметь потенциальные последствия для соблюдения нормативных требований и здоровья населения.
Кроме того, загрязнение может повлиять на точность и воспроизводимость рентгенофлуоресцентных измерений. Если загрязнение происходит во время подготовки нескольких образцов, вариативность результатов может увеличиться, что затруднит сравнение данных по разным образцам. Это может подорвать надежность анализа и поставить под сомнение достоверность любых выводов, сделанных на основе полученных данных.
Заключение
Загрязнение - тихий саботажник рентгенофлуоресцентного анализа, особенно в процессе измельчения. Понимание источников загрязнения, применение эффективных методов предотвращения и осознание последствий загрязнения имеют решающее значение для поддержания точности и надежности результатов рентгенофлуоресцентного анализа. Тщательная подготовка образцов, использование соответствующих материалов и работа в чистой среде позволяют исследователям свести к минимуму риск загрязнения и обеспечить целостность результатов рентгенофлуоресцентного анализа.
Выбор связующего: ключ к однородным гранулам
Выбор подходящего связующего вещества - важнейший аспект подготовки гранул, поскольку он напрямую влияет на однородность и стабильность конечных гранул. Связующие вещества служат "клеем", который удерживает частицы образца вместе, гарантируя, что гранулы останутся целыми во время анализа и не загрязнят спектрометр сыпучим порошком. Чаще всего для приготовления гранул используются смеси целлюлозы и воска, которые известны своей способностью гомогенизироваться с образцом и эффективно связывать частицы в процессе прессования.
Роль связующих веществ в приготовлении гранул
Связующие вещества играют важнейшую роль в процессе гранулирования, способствуя образованию сплошных гранул из порошкового образца. Без связующего вещества частицы образца не будут прилипать друг к другу, что приведет к образованию рыхлых и нестабильных гранул, которые могут легко распасться на части или загрязнить спектрометр. Связующее вещество выступает в качестве среды, которая позволяет частицам слипаться, образуя твердую и однородную гранулу, которую можно анализировать без помех со стороны свободных частиц.
Распространенные типы связующих веществ
Смеси целлюлозы и воска
Смеси целлюлозы и воска являются наиболее широко используемыми связующими при приготовлении гранул благодаря своей эффективности и простоте применения. Эти связующие вещества обычно добавляют к образцу в пропорции 20-30 %, в зависимости от конкретных требований анализа. Целлюлозный компонент обеспечивает структурную целостность гранул, а воск - сцепление частиц друг с другом. Такое сочетание позволяет сформировать однородную гранулу, которая отличается прочностью и стабильностью.

Акриловые связующие
В некоторых случаях акриловые связующие могут использоваться в качестве альтернативы смесям целлюлозы и воска. Однако акриловые связующие могут быть более сложными для гомогенизации с образцом, часто требуя ручного смешивания, а не автоматического добавления во время измельчения. Это может привести к несоответствию конечного гранулята, так как связующее может неравномерно распределиться по образцу.
Предварительно смешанные гранулы
Для удобства некоторые связующие вещества выпускаются в виде предварительно смешанных гранул, которые можно автоматически добавлять в мельницу во время размола. Такие предварительно смешанные гранулы обеспечивают равномерное распределение связующего по образцу, что позволяет получить более стабильные и надежные результаты. Однако выбор гранул для предварительной смеси должен быть тщательно продуман, чтобы убедиться в их совместимости с конкретным анализируемым образцом.
Выбор наиболее подходящего связующего вещества
Выбор наиболее подходящего связующего для конкретного образца зависит от нескольких факторов, включая тип образца, желаемую прочность гранул и специфические требования к анализу. Ниже приведены основные соображения, которые следует учитывать при выборе связующего:
Тип образца
Для разных образцов могут потребоваться разные типы связующих. Например, трудноизмельчаемые образцы или образцы, содержащие трудноизмельчаемые фазы, могут выиграть от использования связующего, которое может лучше гомогенизироваться с образцом и удерживать частицы вместе. В таких случаях смесь целлюлозы и воска может быть более эффективной, чем акриловое связующее.
Прочность гранул
Прочность конечного гранулята - еще один важный момент. Более прочные гранулы с меньшей вероятностью распадутся при обработке или анализе, что снижает риск загрязнения и повышает точность результатов. Смеси целлюлозы и воска обычно предпочтительны из-за их способности создавать прочные и стабильные гранулы.
Требования к анализу
Особые требования к анализу, такие как тип используемого спектрометра или чувствительность измерений, также могут повлиять на выбор связующего. Например, некоторые связующие могут давать более однородные гранулы, которые лучше подходят для анализа с высоким разрешением, в то время как другие могут быть более эффективными для предотвращения загрязнения.
Лучшие практики по выбору связующего
Чтобы добиться наилучших результатов, важно следовать передовым методам при выборе и использовании связующего. Вот несколько советов, которые помогут вам получить максимальную отдачу от связующего:
Протестируйте несколько скоросшивателей
Если возможно, протестируйте несколько связующих с образцом, чтобы определить, какой из них дает наиболее стабильные и надежные результаты. Это поможет вам определить оптимальное связующее для конкретного применения.
Обратите внимание на пропорции
Пропорция связующего к образцу имеет решающее значение для получения однородной гранулы. Слишком большое количество связующего может привести к получению слишком твердых и хрупких гранул, а слишком малое - к слабым и нестабильным гранулам. Обычно рекомендуется соотношение 20-30 % связующего к образцу, но оно может меняться в зависимости от конкретного связующего и образца.
Обеспечьте равномерное распределение
Независимо от типа используемого связующего, важно обеспечить его равномерное распределение по образцу. Этого можно добиться путем тщательного перемешивания связующего с образцом или с помощью предварительно смешанных гранул. Неравномерное распределение связующего может привести к несоответствиям в конечном грануляте, что повлияет на точность анализа.
Контролируйте размер частиц
Размер частиц образца также может влиять на эффективность связующего. Более крупные частицы могут привести к образованию менее однородных гранул, поскольку связующее вещество не сможет эффективно удерживать все частицы вместе. Измельчение образца до однородного размера частиц поможет улучшить эффективность связующего и получить более однородный гранулят.
Заключение
Выбор связующего вещества является критическим фактором при подготовке однородных и стабильных гранул для анализа. Выбрав наиболее подходящее связующее для вашего образца и следуя передовым методам его использования, вы сможете обеспечить стабильность, надежность и отсутствие загрязнений в ваших гранулах. Выбираете ли вы смесь целлюлозы и воска, акриловое связующее или предварительно смешанные гранулы, тщательный учет рассмотренных выше факторов поможет вам добиться наилучших результатов в анализе.
Толщина гранул: Обеспечение достаточной глубины отбора проб
В рентгенофлуоресцентном анализе (XRF) толщина прессованного гранулята является критическим фактором, который напрямую влияет на точность и надежность результатов анализа. Гранулы должны быть достаточно толстыми, чтобы рентгеновское излучение, образующееся в образце, могло выйти из него без повторного поглощения, что позволяет точно измерить содержание элементов. В этом разделе мы рассмотрим важность толщины гранул, способы расчета необходимой толщины и распространенные ошибки, которые могут привести к неточным результатам.

Важность толщины гранул
При подготовке прессованных гранул к рентгенофазовому анализу первоочередной задачей является обеспечение "бесконечной толщины" гранул для всех измеряемых элементов по отношению к рентгеновскому лучу. Это означает, что толщина гранулы должна превышать глубину выхода самого высокоэнергетического элемента в образце. Если гранула слишком тонкая, рентгеновское излучение, созданное в образце, может быть поглощено до того, как оно достигнет детектора, что приведет к занижению концентрации элемента.
Глубина выхода элемента зависит от его энергетического уровня, причем элементы с более высокой энергией обычно имеют большую глубину выхода. Например, элементы с более высокими атомными номерами (например, железо, медь) будут иметь большую глубину выхода по сравнению с элементами с более низкой энергией (например, натрий, магний). Поэтому гранула должна быть достаточно толстой, чтобы вместить самую большую глубину выхода интересующих элементов.
Расчет требуемой толщины окатыша
Чтобы определить требуемую толщину гранул, необходимо учесть глубину выхода самого высокоэнергетического элемента в образце. Глубина выхода может быть рассчитана с помощью коэффициентов массового поглощения элементов, присутствующих в образце. Коэффициент массового поглощения - это мера того, насколько материал может поглощать рентгеновские лучи, и зависит от атомного номера элемента и энергии рентгеновских лучей.
Для практических целей гранулы, изготовленные из 8-10 граммов образца для гранул диаметром 32 мм или 13-15 граммов образца для гранул диаметром 40 мм, обычно достаточны для элементов, которые могут быть измерены даже самыми мощными приборами для волновой дисперсионной рентгеновской флуоресценции (WDXRF), доступными в настоящее время. Эти веса обеспечивают достаточную толщину гранул для предотвращения поглощения рентгеновского излучения и получения точных результатов анализа.
Распространенные ошибки при определении толщины гранул
Одна из наиболее распространенных ошибок при приготовлении прессованных гранул - недооценка необходимой толщины. Это может произойти, если толщина гранулы недостаточна для того, чтобы вместить самую большую глубину выхода измеряемых элементов. В результате рентгеновское излучение, созданное в образце, может быть поглощено, что приведет к неточным измерениям.
Еще одна распространенная ошибка - неучет средней атомной массы образца. Способность образца к поглощению прямо пропорциональна его средней атомной массе, то есть более тяжелые элементы будут поглощать больше рентгеновских лучей, чем более легкие. Поэтому очень важно учитывать средний элементный состав образца при определении необходимой толщины гранул.
Соображения, связанные с загрязнением
Помимо толщины гранул, загрязнение является еще одним важным фактором, который может повлиять на качество рентгенофлуоресцентного анализа. Загрязнение может возникнуть в процессе измельчения пробы и может исходить от устройства для подготовки пробы или перекрестного загрязнения между пробами. Чтобы минимизировать загрязнение, необходимо использовать чистое оборудование и следить за тем, чтобы процесс пробоподготовки проходил в контролируемой среде.
Лучшие практики подготовки гранул
Для обеспечения точного и надежного рентгенофлуоресцентного анализа необходимо следовать передовым методам подготовки гранул. Это включает в себя использование соответствующего количества образца для достижения требуемой толщины гранул, выбор правильного связующего вещества и постоянное давление в процессе прессования. Внимание к деталям и последовательность в процессе подготовки - залог минимизации ошибок и получения высококачественных аналитических результатов.
Таким образом, толщина прессованной гранулы - это критический фактор в XRF-анализе, который напрямую влияет на точность и надежность результатов анализа. Если обеспечить достаточную толщину гранулы, чтобы вместить самую большую глубину проникновения измеряемых элементов, и следовать передовым методам подготовки гранул, аналитики смогут получить точные и достоверные измерения элементов, присутствующих в образце.
Применение давления: Баланс между сжатием и однородностью
Достижение оптимального давления при прессовании гранул имеет решающее значение для создания высококачественных гранул, не содержащих пустот и обладающих постоянными свойствами. Этот процесс включает в себя применение нужного количества давления для сжатия образца и рекристаллизации связующего, что обеспечивает плотность и однородность конечного продукта. В этом разделе рассматриваются важнейшие аспекты применения давления, включая влияние избыточного и недостаточного давления, а также методы достижения оптимального давления для ваших образцов.
Важность правильного применения давления
Основная цель прессования гранул - устранить пустоты внутри гранул, которые могут существенно повлиять на интенсивность светлых элементов в образце. Хорошо спрессованный гранулят должен быть достаточно плотным, чтобы предотвратить появление внутренних пустот, обеспечивая равномерное распределение образца и связующего вещества. Для этого необходимо приложить достаточное давление, чтобы полностью сжать образец и перекристаллизовать связующее вещество.
Эксперименты и оптимальный диапазон давления
Поиск оптимального давления для конкретного образца требует проведения экспериментов. Начните с увеличения давления на образцы и наблюдайте за интенсивностью свечения элементов. Большинство образцов достигают максимальной интенсивности при давлении в диапазоне 25-35 метрических тонн (Т) в течение 1-2 минут. Этот диапазон является хорошей отправной точкой, но отдельные образцы могут потребовать корректировки в зависимости от их специфических свойств.

Избегание избыточного и недостаточного давления
Чрезмерное прессование может привести к ряду проблем, в том числе к превышению предела прочности компакта, что приведет к образованию трещин или "укупорке", когда верхняя часть гранулы отделяется от остальной части. Сверхвысокие усилия не обязательно приводят к улучшению качества гранул и могут принести больше вреда, чем пользы. С другой стороны, недостаточное прессование может оставить пустоты внутри гранул, что приведет к непостоянным результатам и снижению интенсивности для легких элементов.
Техники оптимального применения давления
-
Медленный сброс давления: Приложив необходимое давление, сбрасывайте его медленно, чтобы предотвратить растрескивание поверхности гранул. Быстрый сброс давления может привести к разрушению под напряжением, что нарушит целостность гранулы.
-
Выравнивание и загрузка фильеры: Убедитесь, что пресс и матрица правильно выровнены для равномерного давления. Переполнение гильзы штампа порошком может привести к неравномерному сжатию, поэтому его следует избегать. Нагружайте матрицу не более чем на 50% от предела текучести стали, чтобы не превысить ее возможности.
-
Размеры гранул: Поддерживайте сбалансированное соотношение между высотой и диаметром гранул. Гранулы, длина которых значительно превышает их диаметр, могут испытывать большие напряжения вблизи верхнего плунжера, что может привести к растрескиванию. При прессовании более длинных гранул следует использовать меньшие усилия, смазывать пресс-форму и использовать уплотнительное кольцо между опорной плитой и гильзой для более равномерного распределения напряжений.
-
Равномерное распределение порошка: При перемещении смеси образцов в полость матрицы следите за ее равномерным распределением. Неравномерное распределение может привести к неравномерному сжатию и образованию пустот в гранулах.
Практические шаги по применению давления
- Закрепите пресс для гранул: Закрепите матрицу в полости пресса и убедитесь, что она правильно выровнена.
- Перенесите образец: С помощью металлического шпателя равномерно распределите смесь измельченных образцов в полости пресса.
- Распределите частицы: Вставьте пресс и поверните его, чтобы равномерно распределить частицы.
- Закрепите набор штампов: Установите набор матриц в гидравлический пресс для гранул и плотно закрепите его, вращая колесо.
- Применить давление: Закройте клапан гидравлического пресса и потяните за уровень, чтобы создать давление, пока ручка не станет тугой.
- Отпустить давление: Чтобы освободить матрицу, сначала ослабьте давление, затем поднимите верхнее колесо пресса и, наконец, выньте матрицу.
Тщательно сбалансировав сжатие и однородность, вы сможете получить высококачественные гранулы, отвечающие вашим экспериментальным требованиям. Правильное применение давления в сочетании с вниманием к деталям на каждом этапе процесса гарантирует, что ваши гранулы будут плотными, без пустот и стабильными по свойствам.
Коэффициент разбавления: Тонкая настройка для получения точных результатов
Коэффициент разбавления играет важную роль в рентгенофлуоресцентном анализе (РФА), влияя на точность и надежность результатов. Правильное определение и применение соответствующего коэффициента разбавления необходимо для смягчения общих проблем, связанных с пробоподготовкой и влиянием матрицы, обеспечивая точное представление элементного состава образца.

Влияние коэффициента разбавления на рентгенофлуоресцентный анализ
В рентгенофлуоресцентном анализе коэффициент разбавления напрямую влияет на интенсивность рентгеновских линий, испускаемых образцом. Хорошо подобранный коэффициент разбавления может сбалансировать эффекты матрицы, такие как поглощение и усиление, которые могут исказить измеренную интенсивность. Поглощение происходит, когда элементы в образце поглощают рентгеновские лучи, испускаемые другими элементами, уменьшая интенсивность рентгеновских лучей, попадающих на детектор. Усиление, с другой стороны, происходит, когда присутствие высокоэнергетических элементов в образце возбуждает атомы анализируемого элемента, увеличивая интенсивность рентгеновских линий.
Тщательный подбор коэффициента разбавления позволяет свести к минимуму эти матричные эффекты, что приводит к получению более точных и воспроизводимых результатов. Правильный коэффициент разбавления гарантирует, что образец не будет ни слишком концентрированным, что может привести к чрезмерному поглощению, ни слишком разбавленным, что может привести к слабой интенсивности сигнала, которую трудно точно измерить.
Определение подходящего коэффициента разбавления
Определение подходящего коэффициента разбавления включает в себя сочетание теоретических соображений и практических экспериментов. Цель - получить однородную смесь, в которой образец равномерно распределен по всему материалу матрицы. Вот несколько основных шагов, которые необходимо выполнить:
-
Понять состав пробы: Начните с тщательного изучения элементного состава образца. Это включает в себя знание концентраций основных, второстепенных и следовых элементов. Эта информация очень важна для прогнозирования потенциальных эффектов матрицы.
-
Выберите подходящий матричный материал: Материал матрицы должен быть химически инертным и иметь состав, который минимизирует эффекты поглощения и усиления. Обычно выбирают тетраборат лития (LiBO₂) и борную кислоту (H₃BO₃), которые известны своей способностью образовывать стабильные, однородные смеси с широким спектром типов образцов.
-
Выполните предварительное разбавление: Начните с различных коэффициентов разбавления и измерьте полученную интенсивность. Используйте эти измерения для оценки влияния различных соотношений на интенсивность рентгеновских линий. Ищите соотношение, при котором интенсивность стабильна, а влияние матрицы сведено к минимуму.
-
Оптимизируйте однородность: Убедитесь, что образец тонко измельчен до размера зерна менее 75 мкм. Это необходимо для получения однородной смеси, что очень важно для точного рентгенофлуоресцентного анализа. Чем мельче зерна, тем лучше смесь, тем меньше вероятность образования пустот и неровных поверхностей в конечном грануляте.
-
Оценка стабильности и воспроизводимости: После определения подходящего соотношения разбавления проведите несколько измерений, чтобы убедиться в стабильности и воспроизводимости результатов. Отклонения в результатах могут указывать на проблемы в процессе подготовки образца, такие как неполное смешивание или изменения в размере зерен.
Общие проблемы, связанные с неправильным разбавлением
Неправильное разбавление может привести к нескольким распространенным проблемам в рентгенофлуоресцентном анализе:
-
Чрезмерное поглощение: Слишком концентрированные образцы могут привести к чрезмерному поглощению, когда рентгеновские лучи, испускаемые образцом, значительно поглощаются другими элементами в образце. Это может привести к недооценке концентрации определенных элементов.
-
Слабая интенсивность сигнала: И наоборот, недостаточно концентрированные образцы могут привести к слабой интенсивности сигнала, что затрудняет получение точных измерений. Это особенно проблематично для микроэлементов, которые могут не давать достаточно сильного сигнала для надежного обнаружения.
-
Эффекты матрицы: Неправильное разбавление может усилить эффекты матрицы, такие как поглощение и усиление. Эти эффекты могут исказить измеренную интенсивность, что приведет к неточным результатам.
-
Неоднородные смеси: Если образец не измельчен или соотношение разбавления не оптимизировано, полученная смесь может быть неоднородной. Это может привести к вариациям в измеренных интенсивностях даже в пределах одного образца, что снижает надежность результатов.
Заключение
Точная настройка коэффициента разбавления - важнейший шаг в получении точных и надежных результатов рентгенофлуоресцентного анализа. Тщательно подобрав подходящий коэффициент разбавления и обеспечив однородность смеси, вы сможете свести к минимуму влияние матрицы и другие распространенные проблемы, связанные с подготовкой проб. Такой подход не только повышает точность измерений, но и улучшает воспроизводимость результатов, облегчая сравнение данных по разным образцам и экспериментам.
Перекрестное загрязнение от образца к образцу: Предотвращение интерференции
Перекрестное загрязнение между образцами является критической проблемой в аналитических лабораториях, потенциально приводящей к неточным результатам и нарушению целостности данных. В этом разделе рассматриваются методы минимизации перекрестного загрязнения, важность протоколов очистки и лучшие практики обеспечения чистоты образцов.
Понимание перекрестного загрязнения
Перекрестное загрязнение возникает, когда остатки одного образца мешают анализу другого, что приводит к искажению результатов. Это особенно проблематично в условиях, когда анализируется широкий спектр типов образцов, поскольку риск загрязнения возрастает с увеличением разнообразия образцов. Например, если устройство для подготовки проб, такое как пульверизатор, не очищается тщательно между использованиями, в него могут попасть элементы из одного образца в другой, что исказит результаты анализа.

Методы минимизации перекрестного загрязнения
-
Использование специализированного оборудования: Одним из эффективных методов минимизации перекрестного загрязнения является использование специального оборудования для определенных типов образцов. Это гарантирует, что остатки одного типа образца не будут мешать другому. Например, специальный измельчитель для металлических образцов может предотвратить попадание металлических элементов в неметаллические образцы.
-
Протоколы тщательной очистки: Очень важно внедрить строгие протоколы очистки. После каждого использования оборудование должно очищаться в соответствии со стандартной процедурой. Это включает использование соответствующих растворителей или чистящих средств и обеспечение удаления всех остатков. Например, стальные шлифовальные сосуды следует очищать растворителями, которые могут растворить остатки железа, никеля и хрома.
-
Методы подготовки образцов: Техника, используемая для подготовки проб, также может влиять на риск перекрестного загрязнения. Например, при подготовке калибровочных стандартов для рентгенофлуоресцентного анализа очень важно, чтобы матрица калибровочных стандартов соответствовала матрице образцов. Это помогает повысить точность и снизить риск загрязнения. Кроме того, использование высококачественных калибровочных образцов с сертификатом анализа может обеспечить уверенность в целостности процесса калибровки.
-
Использование держателей образцов: Для небольших и тонких образцов использование держателя образца позволяет предотвратить помехи от объектов, расположенных за образцом. Это обеспечивает более точный анализ за счет сохранения постоянного расстояния между образцом и окном обнаружения спектрометра.
-
Измерение нескольких поверхностей: При исследовании больших металлических образцов для получения более точных результатов рекомендуется проводить измерения нескольких поверхностей несколько раз. Это уменьшает вероятность искажения данных из-за локального загрязнения на одной поверхности.
Важность протоколов очистки
Протоколы очистки являются основой для предотвращения перекрестного загрязнения. Они гарантируют, что оборудование не содержит остатков, которые могут помешать проведению последующих анализов. Регулярное техническое обслуживание и соблюдение стандартных операционных процедур (СОП) при вводе в эксплуатацию и остановке оборудования имеют решающее значение. Например, частые проверки приборов, чтобы убедиться, что они работают как положено, могут предотвратить проблемы, которые могут привести к загрязнению.
Лучшие практики подготовки проб
Основные задачи пробоподготовки включают обеспечение однородности образца и устранение возможных помех, связанных с исходной формой образца. Методы, обеспечивающие преимущества в скорости и количестве образцов, которые можно подготовить за один раз, особенно ценны в лабораториях с высокой пропускной способностью. Однако важно соблюдать баланс между скоростью и необходимостью обеспечения точности и целостности.
Заключение
Предотвращение перекрестного загрязнения проб жизненно важно для поддержания точности и надежности аналитических результатов. Внедряя специальное оборудование, строгие протоколы очистки и передовые методы подготовки проб, лаборатории могут значительно снизить риск контаминации. Это не только обеспечивает целостность отдельных анализов, но и способствует общему доверию к данным лаборатории.
Лучшие практики по снижению ошибок при подготовке гранул для рентгенофлуоресцентного анализа
Когда речь идет о рентгенофлуоресцентном анализе (РФА), качество результатов в значительной степени зависит от подготовки образцов. Процесс создания прессованных гранул для рентгенофлуоресцентного анализа - это критический этап, который может существенно повлиять на точность и достоверность данных. Чтобы свести к минимуму ошибки и обеспечить стабильность результатов, важно следовать передовым методам подготовки гранул для рентгенофлуоресцентного анализа. В этом разделе описаны основные стратегии разработки методов, внимание к деталям и последовательность действий для уменьшения ошибок при подготовке проб для рентгенофлуоресцентного анализа.
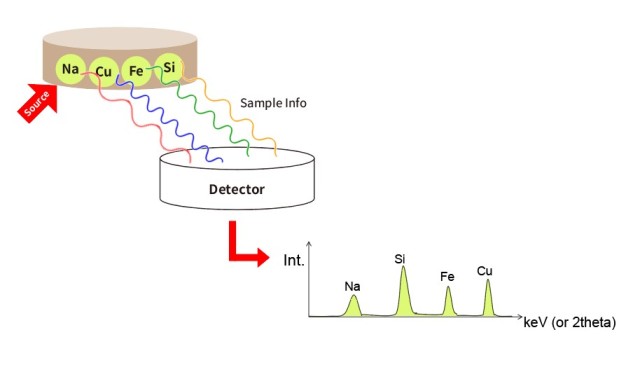
Разработка метода
Первым шагом к снижению ошибок при подготовке гранул для рентгенофлуоресцентного анализа является разработка надежного метода. Для этого необходимо понять специфические требования к анализируемому образцу и соответствующим образом настроить процесс подготовки. Диапазон типов образцов для рентгенофлуоресцентного анализа огромен, и каждый образец может обладать уникальными характеристиками, требующими корректировки метода подготовки. Например, размер частиц образца, выбор связующего вещества и коэффициент разбавления - критические факторы, которые необходимо тщательно учитывать.
-
Размер частиц: Размер частиц образца может существенно повлиять на однородность гранул и, следовательно, на точность рентгенофлуоресцентного анализа. Обычно рекомендуется использовать частицы размером менее 100 микрометров, чтобы обеспечить равномерное распределение образца в грануле. Более крупные частицы могут привести к неоднородности, что приведет к расхождению показаний.
-
Выбор связующего вещества: Связующее вещество, используемое в процессе приготовления гранул, играет решающую роль в обеспечении целостности и стабильности гранул. К распространенным связующим веществам относятся боратные стекла, целлюлоза и воски. Выбор связующего вещества должен основываться на совместимости с образцом и специфических требованиях рентгенофлуоресцентного анализа. Например, некоторые связующие могут содержать элементы, которые могут помешать анализу, поэтому важно выбрать связующее, которое минимизирует такие помехи.
-
Коэффициент разбавления: Коэффициент разбавления означает соотношение образца и связующего вещества. Правильный коэффициент разбавления обеспечивает равномерное распределение образца в гранулах, что снижает риск пере- или недопредставления определенных элементов. Оптимальный коэффициент разбавления зависит от состава образца и аналитических требований. Часто необходимо провести серию тестов, чтобы определить оптимальный коэффициент разбавления для конкретного образца.
Внимание к деталям
Внимание к деталям имеет первостепенное значение при подготовке гранул для XRF, чтобы свести к минимуму ошибки. Небольшие отклонения в процессе подготовки могут привести к значительным расхождениям в результатах анализа. Поэтому очень важно соблюдать последовательность на каждом этапе подготовки.
-
Применение давления: Величина давления, прилагаемого в процессе прессования гранул, является критическим фактором, влияющим на плотность и однородность гранул. Недостаточное давление может привести к образованию рыхлых гранул, а избыточное - к неравномерному сжатию образца. Оптимальное давление должно определяться исходя из свойств образца и конкретных требований рентгеноструктурного анализа. Постоянство прилагаемого давления необходимо для обеспечения воспроизводимых результатов.
-
Толщина гранулы: Толщина конечной гранулы может повлиять на чувствительность и точность рентгенофлуоресцентного анализа. Слишком тонкая гранула может не обеспечить достаточного сигнала, а слишком толстая гранула может привести к эффекту поглощения, который исказит результаты. Идеальная толщина гранул обычно составляет от 1 до 3 миллиметров, в зависимости от состава образца и аналитических требований.
-
Перекрестное загрязнение между образцами: Перекрестное загрязнение между образцами - распространенный источник ошибок при рентгенофлуоресцентном анализе. Чтобы свести этот риск к минимуму, необходимо тщательно очищать все оборудование и поверхности между пробоподготовками. Это касается пресса для гранул, контейнеров для образцов и любых других инструментов, используемых в процессе. Кроме того, использование специальных инструментов для каждого образца может еще больше снизить риск перекрестного загрязнения.
Последовательность
Последовательность в процессе подготовки является ключевым фактором для снижения ошибок при проведении рентгенофлуоресцентного анализа. Для этого необходимо разработать стандартные операционные процедуры (СОП) и проводить регулярные проверки контроля качества (КК) и обеспечения качества (ОК).
-
Стандартные операционные процедуры (СОПы): Разработка и соблюдение СОПов обеспечивает последовательное выполнение каждого этапа процесса подготовки. СОПы должны включать подробные инструкции по подготовке проб, включая размер частиц, выбор связующего, коэффициент разбавления, применение давления и толщину гранул. Регулярное обучение и документирование процесса подготовки может помочь обеспечить последовательное соблюдение СОП всеми сотрудниками.
-
Контроль качества (QC) и обеспечение качества (QA): Процедуры QC и QA необходимы для контроля точности и надежности рентгенофлуоресцентного анализа. Они включают использование сертифицированных стандартных образцов (СО), холостых образцов, дубликатов и реплик для проверки точности результатов. Регулярные проверки КК помогут выявить любые отклонения от ожидаемых результатов и своевременно внести коррективы в процесс подготовки.
-
Сопоставление матриц: Подбор матрицы предполагает подготовку образцов, сходных по составу с неизвестными образцами, чтобы свести к минимуму влияние матрицы. Это поможет уменьшить ошибки, вызванные различиями в составе образца, такими как эффекты поглощения или усиления. Подбор матрицы особенно важен при анализе сложных образцов с разным составом.
Заключение
В заключение следует отметить, что сокращение количества ошибок при подготовке гранул для рентгенофлуоресцентного анализа требует сочетания разработки методов, внимания к деталям и последовательности. Тщательный учет таких факторов, как размер частиц, выбор связующего, коэффициент разбавления, давление и толщина гранул, а также разработка СОПов и регулярное проведение контроля качества и ОТК позволяют минимизировать ошибки и обеспечить точные и надежные результаты XRF-анализа. Ключ к успешному проведению XRF-анализа лежит в тщательной подготовке образцов, поскольку даже небольшие отклонения могут привести к значительным расхождениям в конечных результатах.
Заключение: Достижение точности в рентгенофлуоресцентном анализе
Мастерство подготовки образцов для рентгенофлуоресцентного анализа на прессе PELLET PRESS имеет решающее значение для получения точных результатов. Решив такие общие проблемы, как размер частиц, загрязнение, выбор связующего, толщина гранул, давление, коэффициент разбавления и перекрестное загрязнение, вы сможете значительно повысить точность рентгенофлуоресцентного анализа. Соблюдение передового опыта и тщательное внимание к деталям гарантирует, что каждый аспект подготовки гранул будет оптимизирован, что в конечном итоге приведет к надежным и стабильным результатам анализа. Вкладывая время в совершенствование методов подготовки, вы получите более точные данные и более глубокое понимание образцов.
Связанные товары
- Автоматический лабораторный гидравлический пресс для таблеток XRF и KBR
- Лабораторная пресс-форма для таблетирования порошка в пластиковом кольце XRF & KBR для ИК-Фурье
- Лабораторная пресс-форма для таблетирования порошка в стальном кольце XRF & KBR для ИК-Фурье
- Лабораторная пресс-форма для таблеток из борной кислоты для рентгенофлуоресцентного анализа
- Лабораторный гидравлический пресс для таблеток для применений XRF KBR FTIR
Связанные статьи
- Эксплуатация автоматического лабораторного пресса для гранулирования XRF
- Понимание холодного изостатического прессования (CIP) и его преимуществ
- Понимание технических аспектов холодного изостатического прессования
- Руководство для Xrf Pellet Press
- Подробное руководство по прессованию таблеток РФА с использованием автоматического гидравлического пресса KinTek